Methylene Chloride
The U.S. Environmental Protection Agency (EPA) has issued a final rule (PDF) regulating the use of the solvent methylene chloride (dichloromethane, DCM). The EPA has determined that DCM represents an “unreasonable risk of injury to health” and the rule bans almost all uses of the chemical. Because of this final rule, colleges and universities are required to comply with this new law and the resulting regulations. Fact Sheet: EPA Regulation of Methylene Chloride under TSCA (PDF).
What that means for ECU labs is that while the laboratory use of methylene chloride is not banned, the EPA has issued strict requirements for any continued use, even if performed in a certified fume hood.
EH&S strongly urges you to consider not using methylene chloride by:
- Elimination: If you no longer need the chemical submit for hazardous waste pickup request immediately.
- Substitution: If you actively use the chemical or products containing .0.1% DCM, consider the alternatives and if they will work for your project.
- American Chemical Society DCM Alternatives and Resources
- Methylene Chloride Replacements: Green Chemistry Teaching and Learning Community (XLSX)
- UPenn Fact Sheet: Solvent Alternatives
- Peptide Synthesis: 2-methyl tetrahydrofuran (2-MeTHF) or Ethyl acetate
- Extractions or Chromatography: Ethyl acetate, Heptane, Toluene, 2-MeTHF, Methyl tert-butyl ether
- Chromatography Separations: Cyclopentyl methyl ether, or mixtures like dimethyl carbonate and methanol or ethyl acetate and isopropanol; can use reverse phase chromatography instead
- Green Chromatography Solvent Mixtures: eluents and mixtures with heptanes, methyl tert-butyl ether, methanol, ethanol, ethyl acetate, and isopropanol for separations involving neutral, acidic, and basic compounds
- Replacements in Reactions: benzo trifluoride for Dess-Martin/Swern, Sakurai, Friedel-Crafts, and Diels-Alder reactions; 2-methyltetrahydrofuran for reductive amination; dimethyl carbonate for methylation and carbonylation reactions
If you choose to continue to use methylene chloride, you are required to take additional steps. You will be required to fund extensive exposure monitoring and develop several detailed safety plans to prevent exposures. A Guide to Complying with the Methylene Chloride Regulation (PDF)
EH&S will assist with developing a Workplace Chemical Protection Program (WCPP) that protects people from unreasonable risk posed by occupational exposures from certain conditions of use. The WCPP will include the following:
- Initial monitoring to determine the frequency of periodic monitoring Periodic monitoring every 3 months, 6 months, or 5 years, based on ECEL, ECEL action level, and EPA STEL (department responsible for funding)
- Requirements to reduce exposures based on the NIOSH hierarchy of controls (department responsible for funding additional control measures)
- Identification of regulated areas
- Exposure Control Plan: documents specific actions taken to mitigate occupational exposures and comply with the WCPP
- Respirator and additional PPE selection criteria to protect workers from any remaining risks (department responsible for funding)
- Comprehensive training program, includes specific job tasks and PPE requirements
- Recordkeeping
Please note the EPA established deadlines. 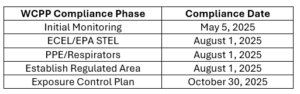
Contact EH&S at safety@ecu.edu PRIOR to purchasing methylene chloride or any products or kits containing methylene chloride.
If you utilize methylene chloride within your laboratory, please ensure that it is listed on your chemical inventory. Also check any other products or kits that may contain methylene chloride as a component. Notify EH&S at safety@ecu.edu.
If you currently have methylene chloride in your laboratory and do NOT use it, please submit a hazardous waste pickup request and remove it from your chemical inventory. Submit a hazardous waste pickup request for any products that contain methylene chloride. Any non-research use of methylene chloride is prohibited.
Methylene Chloride Online Training Course (Coming Soon)